Aerogels are materials in gel form in which the liquid state has been fully substituted with a gaseous state. As a result, these substances have a record-low density along with a number of other unique properties: they are highly resistant (aerogel can handle a load 2,000 times its own weight), exhibit extremely low heat conductivity and have a high surface area. Aerogels are used to produce heat insulation materials for industrial use. They also possess a number of useful biological properties, which makes them a promising material for use in biomedicine.
One of the key topics in this field today is the search for new luminescent agents that would be used in biovisualization and early diagnostics. Luminescence functions of most materials currently in use are based on the regular mechanism: short wavelength radiation is absorbed and then emitted on the long-wavelength part of the spectrum.
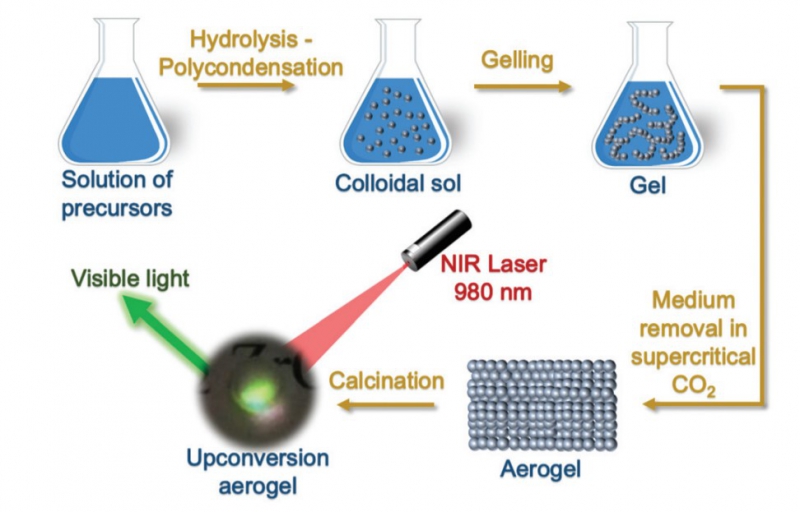
But it is possible to create a completely new class of materials capable of converting infrared radiation into visible radiation using the upconversion process. In this case, the conversion takes place due to the fusion of two or more low-energy longwave photons into a single photon with a higher energy charge.
When developing such materials, researchers use various rare-earth elements. This is a complicated process that involves several stages of synthesis. Researchers at ITMO University’s international laboratory SCAMT have developed a simple, all-purpose method for the production of metal oxide-based (oxides of zirconium, hafnium, and tantalum) upconversion aerogels doped with the rare-earth elements erbium and ytterbium.
Tailor-made conditions of the synthesis have allowed the scientists to produce materials with an even distribution of integrated dopants (modifying additives necessary to enhance a material with optical properties) and unique luminescent properties.

“It is well-known that luminescent aerogels can be produced using rare-earth elements. But that requires the creation of a hybrid material that has two layers. We, however, are the first to have integrated the ions of rare-earth elements into the oxide lattice and have thus produced a lithified upconversion aerogel. During spectral analysis, you can only see a single uncontaminated oxide layer,” says Grigoriy Kiselev, lead author of the article and a researcher at SCAMT.
Initially, the researchers had produced a luminescent zirconium oxide by doping its matrix with rare-earth elements and then developed an aerogel based on that material. After developing their technology further, they used it to produce aerogels of other materials using the same method. Researchers at SCAMT have successfully applied this method to oxides of hafnium and tantalum and demonstrated the possibility of obtaining luminescent aerogels based on these materials. In the future, note the article’s authors, this method may be applied to other oxides.
“The combination of such properties as biocompatibility, high surface area, and upconversion luminescence makes possible a wide range of applications for these materials, from optics and photonics to medicine,” adds Grigoriy Kiselev.

Above all, the use of such materials is safe for humans: infrared radiation can penetrate biological tissue deeply without damaging it. In addition, infrared excitation minimizes the autoluminescence of tissue, thus increasing the accuracy of analysis. All this makes such materials highly promising for application as, for instance, luminescent probes in biological research or in fluorescence diagnostics and cancer treatment.
This research was carried out with the support of the Russian Foundation for Basic Research, grant No. 18-29-11078.
Reference: Upconversion Metal (Zr, Hf, Ta) Oxide Aerogels, Kiselev Grigorii; Kiseleva Aleksandra; Ilatovskii Daniil; Koshevaya Ekaterina; Nazarovskaia Daria; Gets Dmitry; Vinogradov Vladimir; Krivoshapkin Pavel; Krivoshapkina Elena, Chemical Communications, DOI: 10.1039/c9cc02452b, 2019.